
QS, quinacrine sterilization, has been available for more than four decades and safely used by 200,000 women in over 50 countries with no reported deaths or serious complications when administered with the approved pellet method. The Center for Disease Control (CDC) reports surgical tubal ligation (STL) has 3.76 deaths/100,000 women treated. QS is safer and 7 times cheaper than STL. Why is it not approved?
QS is voluntary for women who wish to have no more children
QS doesn’t require anesthesia
QS is safer than surgical tubal ligation
QS is as effective as surgical tubal ligation
QS doesn’t cause cancer any more than other methods
QS doesn’t cause tubal pregnancies any more than other methods
QS is more natural than other methods
QS is administered by healthcare pros not only doctors
QS is cheaper than other methods
QS is faster (during lunch break) than other methods
QS is the last contraceptive method a woman will need
QS may reduce maternal death in childbirth
QS may lead to higher quality of life for families
QS may reduce abortions performed worldwide
QS may lead to human population stabilization
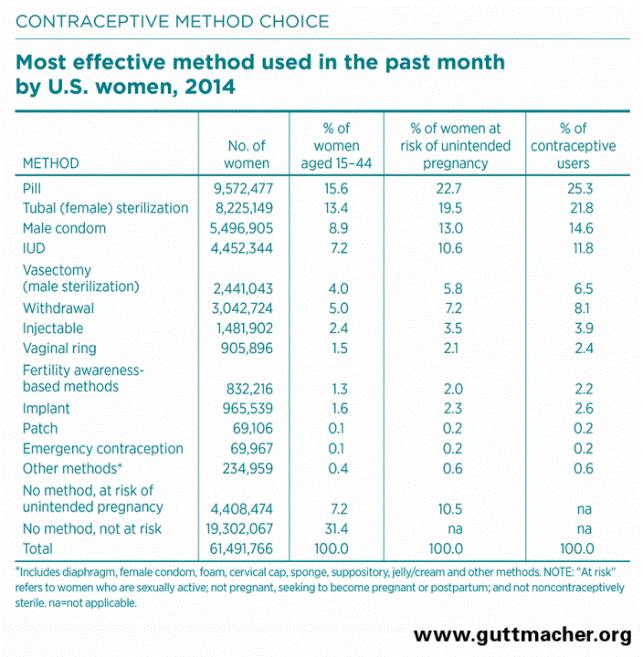
Guttmacher Institute, a leading research and policy organization committed to advancing sexual and reproductive health and rights in the United States and globally, says, “The pill and female sterilization have been the two most commonly used [contraception] methods since 1982.” See their chart below.
In the nonsurgical QS procedure, a healthcare professional inserts 7 pellets, 36 milligrams each, of quinacrine into a woman’s uterus (without anesthesia) using a modified IUD inserter in two doses four weeks apart. That is a total of 504 milligrams compared to the 36,500 – 52,000 milligrams of quinacrine that millions of Americans have safely taken by mouth for a period of one year to combat giardia.
QS costs less than $1 per dose and has been administered by 1600 different trained healthcare professionals usually nurses or midwives. The procedure, most often recommended by word of mouth by women who’ve had it, has been in the public domain for decades so is not patentable. Tens of thousands of women have been studied over decades leading to scores of peer reviewed scientific articles that attest to the QS method’s safety and efficacy which can be found on our website.
ISAF spent over $8 million seeking FDA approval to conduct a Phase III trial (halted in 2007) confirming QS method’s already sterling record and to make it available to women and families around the world who wish to have no more children. FDA staff repeatedly ignored renowned expert scientists and after protracted negotiations denied ISAF to conduct a Phase III trial the latest time in December 2016.
Unfortunately, QS has been attacked by many who recognize its safety, efficacy, and efficiency. There is much written on Wikipedia about compulsory sterilization which QS is not. QS is voluntary as women are first educated about QS and then must sign a consent form. Please lend your voice to support QS on quinacrine.org so we can bring this cheap, safe, and effective method to all women of the world in 2020.
![]()
Bernie Sanders Abortion Rights Help Curb Population Growth to Combat Climate Change
Bernie Slammed For Overpopulation Remarks The View
ISAF Introduces QS 2016

Recent Comments